The outbreak of the COVID-19 pandemic has led industry leading CROs to rethink the way clinical trials are managed. Efficient trial monitoring, while ensuring patient’s safety has been an ongoing challenge for the companies since the time the contagion started spreading at an alarming rate and lockdowns became the new normal. Additionally, proactive strategies against the global health threat have taken a toll on clinical trials. As of March 26, at least 18 pharma or biotech companies have reported clinical trial disruption due to the spread of the pandemic. In addition to existing trials, the efforts to develop vaccines and therapies to counter the COVID-19 pandemic have also been hampered.
Here’s a brief overview of the impact of COVID-19 on clinical trials.
Challenges faced during the COVID-19 catastrophe
The critical first step to combat a disease is gaining precise insights about the patient, their demographics, and the country-level impact of the crisis on running effective clinical trials. Real-time data and detailed reporting are essential to extract insights accurately and consider solutions quickly. However, mobility and travel restrictions imposed to contain the spread of the COVID-19 contagion have hindered Pharmaceutical Companies and CROs from gleaning real-time insights now. Additionally, supply chain disruptions have aggravated the situation and created obstacles not only for new, but for existing clinical trials as well.

According to a study conducted by Continuum Clinical on March 2020, 39% of US sites surveyed state that patients’ interest to enrol in new clinical research trials is likely to go down. Apart from mobility and travel restrictions, the contamination risks at healthcare facilities have stirred up high levels of anxiety and hesitancy among people, leading to increased patient dropouts from clinical trials.
Medidata, an American technology company that provides SaaS for clinical trials, conducted a recent analysis of enrolment data from nearly 4,600 current clinical trials and more than 182,000 study sites across the globe. The analysis reveals a significant decrease in the number of patients entering clinical trials. There was a 65% decrease in year-over-year new patient enrolment during March.
IQVIA, the Human Data Science Company, revealed that 65% of its global sites were impacted in some way by the COVID-19 crisis while 80% of its clinical research sites are inaccessible due to travel restrictions.
The unprecedented circumstance and the sudden need for remote delivery capabilities have put the healthcare industry and pharmaceutical companies under immense pressure and forced them to rethink their approach towards clinical trials and drug development, manufacturing & distribution.
Workarounds to continue Clinical trials amidst the COVID-19 crisis
Some data is better than no data and digital systems are the best fit to collate data remotely. The COVID-19 pandemic has compelled the industry to carry out virtual clinical trials. Research organizations are working on deploying trial models with full digital support that could enable them to recruit and retrieve patients remotely. In fact, many companies are turning to telehealth and other digital systems to help them stay afloat and mitigate test delays.

Niklas Morton, PPD’s senior vice president of digital services, stated that “the pandemic’s impact has created the need for expanded use of digitally enabled trials.” Even more so, the contract research organization has invested and collaborated with virtual trial specialists Medable and Science 37 to mitigate study challenges and support its biopharma clients’ R&D.
The other workaround is deploying in-home clinical services to ensure the safety of patients / trial subjects. Regulatory agencies are backing this solution of bringing trials to the subject. As a matter of fact, the FDA has published a report on March 2020 – Guidance for Conducting Clinical Trials during COVID-19, emphasizing the need for alternative methods in conducting the clinical trials owing to the risks of the contagion. Clinical trials carried out by well-trained healthcare professionals at the subjects’ home ensure safety, compliance, and data collection. In-home services can also help reduce patient dropouts and enhance retention.
How will this impact the Pharmaceutical Value Chain?
The pandemic will have a profound impact on development, approval, manufacturing and distribution of drugs across the globe. The cascade effect of disruptions in clinical trials, will have major implications across the value chain.
The teething problems in adoption of new protocol will cause operational issues with clinical trials, extending their duration and impacting reliability of results. Adoption of new digital systems will require personnel with better ability to adapt themselves. New, enhanced IT systems that facilitate in-home trials will start emerging, leading to a rise in a new product segment. Costs of moving trials to new protocol will result in increase in overall cost, or in-home trials may bring a cost benefit in the long run. The prices of drugs will surely be impacted by this.
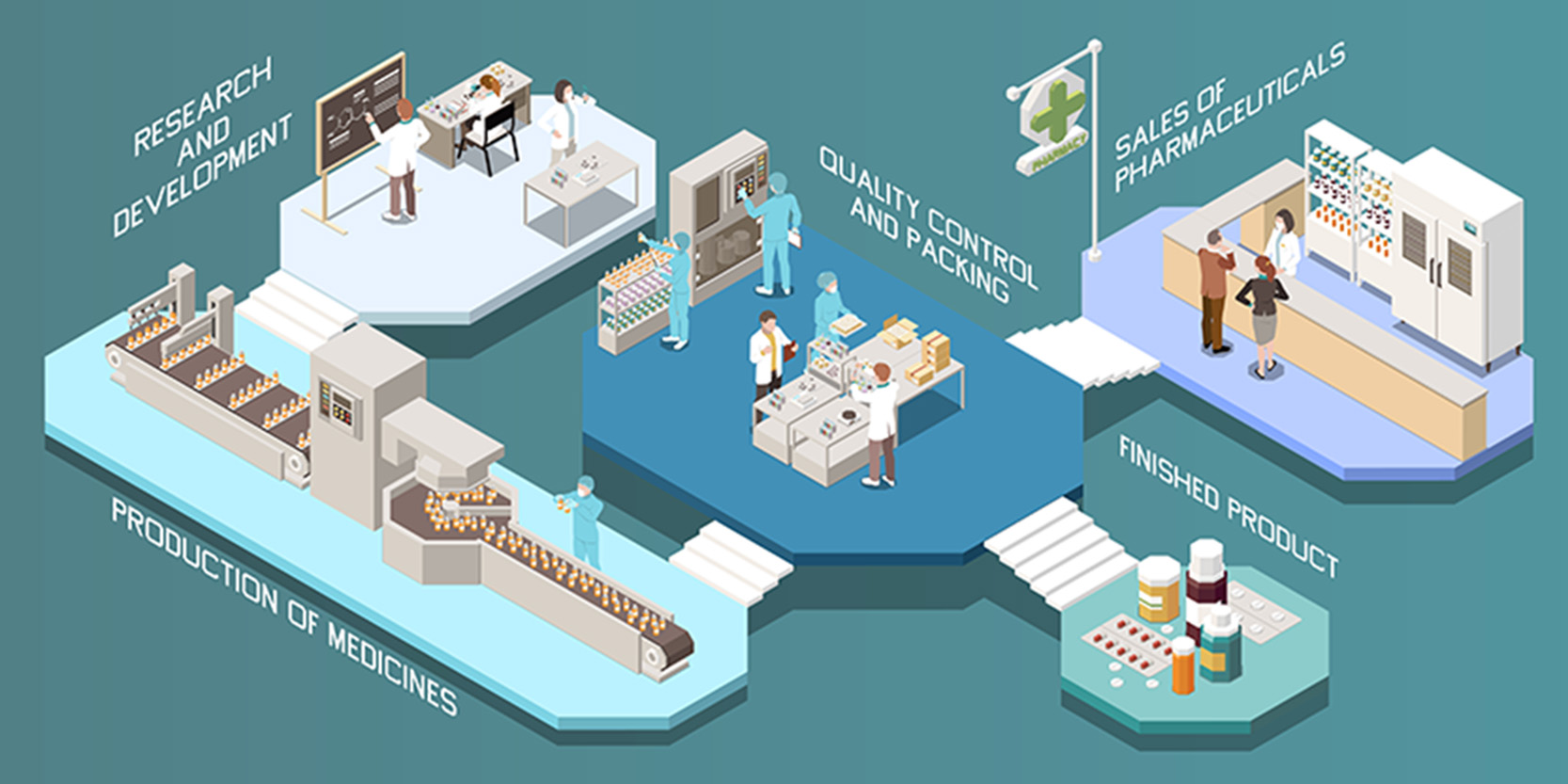
The most critical impact will be on time to market for any given drug. Even new protocols will require further innovation to achieve high throughput rates and expedite ROI on the R&D spend on drugs.
SG Analytics as leading research and analytics service provider is constantly tracking developments in the space to deliver real time insight to its customers. Get in touch with us to know more about the impact of COVID-19 across the Pharmaceutical value chain.